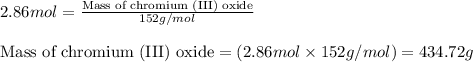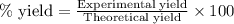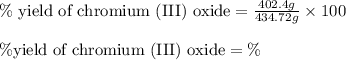Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr02 —2Cr2O3 +02 The reaction of 480.1 g of CrO2 yields 402.4 g of Cr203. Calculate the theoretical yield of Cr203 (assuming complete reaction) and its percentage yield. Theoretical yield = Percentage yield = Submit Answer 2 question attempts remaining

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1
You know the right answer?
Chromium(III) oxide can be prepared by heating chromium(IV) oxide in vacuo at high temperature: 4Cr0...
Questions




History, 08.06.2020 23:57




English, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57


Mathematics, 08.06.2020 23:57



English, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

Chemistry, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

Mathematics, 08.06.2020 23:57

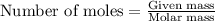 .....(1)
.....(1)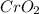 = 480.1 g
= 480.1 g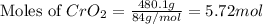
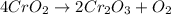
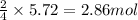 of chromium (III) oxide
of chromium (III) oxide