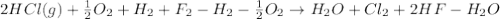Chemistry, 19.02.2020 02:23 Javanese5987
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -202.4 kJ/mol 1/2 H2 (g) + ½ F2 (g) → HF (l) ∆H = -600.0 kJ/mol H2 (g) + ½ O2 (g) → H2O (l) ∆H = -285.8 kJ/mol
Calculate ∆Hrxn for 2 HCl (g) + F2 (g) → 2 HF (l) + Cl2 (g) Just input a number.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3


Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 19:30
If the standard reduction potential of a half-cell is positive, which redox reaction is spontaneous when paired with a hydrogen electrode? reduction both reduction and oxidation neither reduction nor oxidation oxidation
Answers: 1
You know the right answer?
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
Questions


Mathematics, 09.11.2020 20:20


Mathematics, 09.11.2020 20:20


Mathematics, 09.11.2020 20:20




Biology, 09.11.2020 20:20

History, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20


English, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20

Chemistry, 09.11.2020 20:20




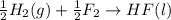 ΔH=-600.0 KJ/mol
ΔH=-600.0 KJ/mol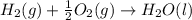 ΔH= -285.8 KJ/mol
ΔH= -285.8 KJ/mol