
Chemistry, 19.02.2020 02:45 blayneaafedt
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and 54 Cr . The 52 Cr isotope has a natural abundance of 83.79 % and an atomic mass of 51.9405 u. The 54 Cr isotope has a natural abundance of 2.37 % and an atomic mass of 53.9389 u. The natural abundances of the 50 Cr and 53 Cr isotopes exist in a ratio of 0.4579 : 1 , and the 50 Cr isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 53 Cr isotope. atomic mass of 53 Cr:.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and...
Questions

Mathematics, 25.06.2019 03:30

English, 25.06.2019 03:30


Mathematics, 25.06.2019 03:30

Chemistry, 25.06.2019 03:30


History, 25.06.2019 03:30

English, 25.06.2019 03:30






Physics, 25.06.2019 03:30

History, 25.06.2019 03:30


Mathematics, 25.06.2019 03:30


Mathematics, 25.06.2019 03:30

Mathematics, 25.06.2019 03:30

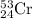 isotope is 52.8367 amu
isotope is 52.8367 amu![_{24}^{50}\text{Cr}\text{ and }_{24}^{53}\textrm{Cr}\text{ isotopes}=[100-(83.79+2.37)]=13.84\%](/tpl/images/0515/1858/e24f2.png)
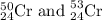 isotopes = 0.4579 : 1
isotopes = 0.4579 : 1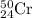 isotope =
isotope = 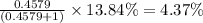
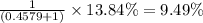
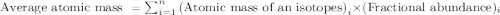 .....(1)
.....(1)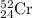 isotope:
isotope: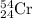 isotope:
isotope:![51.9961=[(49.9460\times 0.0437)+(51.9405\times 0.8379)+(x\times 0.0949)+(53.9389\times 0.0237)]\\\\x=52.8367amu](/tpl/images/0515/1858/822e6.png)


