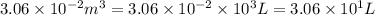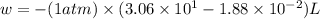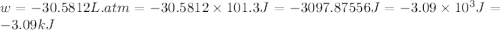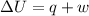Chemistry, 18.02.2020 02:18 emily12403
At 373.15K and 1 atm, the molar volume of liquid water and steamare 1.88 X 10-5 m3 and 3.06 X 10-2m3, respectively. Given that the heat of vaporization ofwater is 40.79 kJ/mol, calculate the values of ?H and ?Ufor 1 mole in the following process:
H2O (l, 373.15 K, 1 atm) ---> H2O(g, 373.15 K, 1 atm)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1


Chemistry, 23.06.2019 15:30
Select the correct answer.the gas in a sealed container has an absolute pressure of 125.4 kilopascals. if the air around the container is at a pressure of 99.8 kilopascals, what is thegauge pressure inside the container?
Answers: 3
You know the right answer?
At 373.15K and 1 atm, the molar volume of liquid water and steamare 1.88 X 10-5 m3 and 3.06 X 10-2m3...
Questions





English, 29.01.2022 21:40





Mathematics, 29.01.2022 21:40

Mathematics, 29.01.2022 21:40



Mathematics, 29.01.2022 21:50


Mathematics, 29.01.2022 21:50




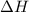 and
and 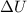 is, 40.79 kJ and 37.7 kJ respectively.
is, 40.79 kJ and 37.7 kJ respectively.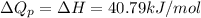
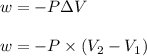
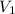 = initial volume =
= initial volume = 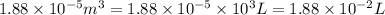
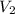 = final volume =
= final volume =