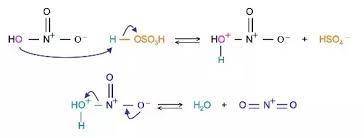
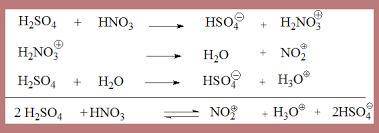
Electrophiles for the electrophilic aromatic substitution reactions have to be very strong to react with the stable aromatic rings. A nitronium ion is needed for nitration of aromatic rings. Complete the mechanism of the formation of the nitronium ion from concentrated nitric acid in concentrated sulfuric acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
Electrophiles for the electrophilic aromatic substitution reactions have to be very strong to react...
Questions



Spanish, 03.08.2019 07:30



Mathematics, 03.08.2019 07:30

History, 03.08.2019 07:30

Business, 03.08.2019 07:30

Health, 03.08.2019 07:30




Geography, 03.08.2019 07:30






Business, 03.08.2019 07:30