Chemistry, 17.02.2020 17:27 hiplikedyani
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to form Al2O3(s) at 25°C and 1 atm. ΔHfAl2O3(s) = −1676 kJ/mol HINT: What does ΔHfAl2O3(s) mean?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

You know the right answer?
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to fo...
Questions

History, 12.12.2020 16:40


History, 12.12.2020 16:40

Business, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

English, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40



Mathematics, 12.12.2020 16:40



Chemistry, 12.12.2020 16:40

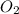 .
.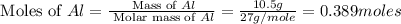
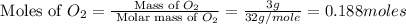
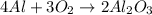
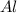
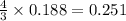 moles of
moles of 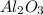
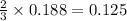 moles of
moles of