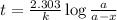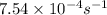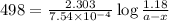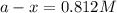Chemistry, 17.02.2020 16:38 dragaozin4505
The rate constant for a first order reaction with a single reactant is 7.54x10-4 s-1. If the initial reactant concentration is 1.18 M, what will the reactant concentration be after 8.30 minutes?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2
You know the right answer?
The rate constant for a first order reaction with a single reactant is 7.54x10-4 s-1. If the initial...
Questions


English, 22.12.2019 16:31


Biology, 22.12.2019 16:31


History, 22.12.2019 16:31




English, 22.12.2019 16:31


History, 22.12.2019 16:31


Mathematics, 22.12.2019 16:31



Spanish, 22.12.2019 16:31

Mathematics, 22.12.2019 16:31

History, 22.12.2019 16:31

Social Studies, 22.12.2019 16:31