
Chemistry, 16.02.2020 07:32 zuleromanos
Aqueous hydrochloric acid (HCI) will react with sodium hydroxide (NaOH) to produce and liquid water (H2O). Suppose 1.1 grams of hydrochloric acid is mixed with 0.420 grams of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Round your answer to 3 significant digits

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Aqueous hydrochloric acid (HCI) will react with sodium hydroxide (NaOH) to produce and liquid water...
Questions




















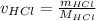
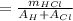
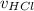 = 0.030 moles.
= 0.030 moles.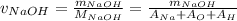
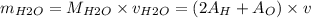
 1 + 16)
1 + 16) 

