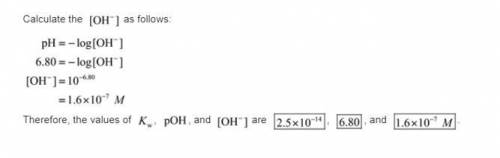. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endothermic, how does Kw change with rising T? Explain with a reaction that includes heat as a reactant or product. (b) In many medical applications, the value of Kw at 37°C (body T) may be more appropriate than the value at 25°C, 1.0x10-14. The pH of pure at 37°C is 6.80. Calculate Kw, pOH and [OH-] at this T.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

You know the right answer?
. Like any equilibrium constant, Kw, changes with temperature. (a) Given that autoionization is endo...
Questions


History, 30.11.2021 04:20



Mathematics, 30.11.2021 04:20

Mathematics, 30.11.2021 04:20



Mathematics, 30.11.2021 04:20


History, 30.11.2021 04:20



Mathematics, 30.11.2021 04:20

Mathematics, 30.11.2021 04:20


Mathematics, 30.11.2021 04:20


Mathematics, 30.11.2021 04:20

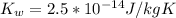
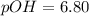
![[OH^{-}] =1.6*10^{-7} M](/tpl/images/0511/5212/a2f6d.png)