Chemistry, 14.02.2020 02:48 seiglersteven99
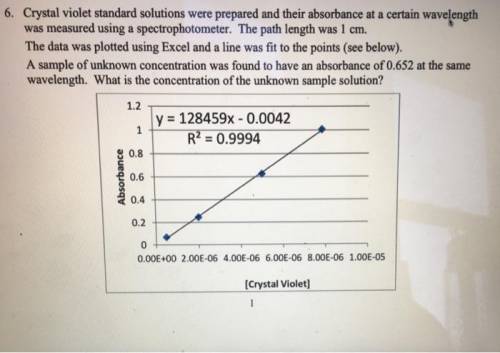
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was measured using a spectrophotometer. The path length was 1 cm. The data was plotted using Excel and a line was fit to the points (see below). A sample of unknown concentration was found to have an absorbance of 0.652 at the same wavelength. (a) What is the molar absorptivity of this compound at the certain wavelength? (b) What is the concentration of the unknown sample solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Crystal violet standard solutions were prepared and their absorbance at a certain wavelength was mea...
Questions

History, 18.07.2019 04:00

Biology, 18.07.2019 04:00


Mathematics, 18.07.2019 04:00


Mathematics, 18.07.2019 04:00

History, 18.07.2019 04:00



Mathematics, 18.07.2019 04:00


Physics, 18.07.2019 04:00

Arts, 18.07.2019 04:00


Physics, 18.07.2019 04:00



Business, 18.07.2019 04:00

History, 18.07.2019 04:00