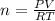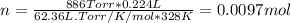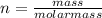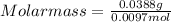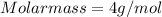Chemistry, 12.02.2020 20:36 kiannadgarnica
A sample of gas has a mass of 38.8 mg. Its volume is 224 mL at a temperature of 55 °C and a pressure of 886 torr. Find the molar mass of the gas.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
A sample of gas has a mass of 38.8 mg. Its volume is 224 mL at a temperature of 55 °C and a pressure...
Questions


Mathematics, 14.04.2021 17:40




Mathematics, 14.04.2021 17:40





Mathematics, 14.04.2021 17:40

Mathematics, 14.04.2021 17:40


Physics, 14.04.2021 17:40


Mathematics, 14.04.2021 17:40

Chemistry, 14.04.2021 17:40


Biology, 14.04.2021 17:40