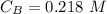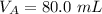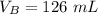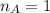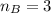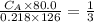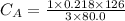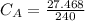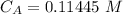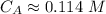Chemistry, 12.02.2020 20:06 liloleliahx2
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH solution. What is the concentration of the H3PO4 solution (in M)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 15:30
How many grams of c3h8 is needed in the reactants to produce 10.5 mil of h2o
Answers: 2
You know the right answer?
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 M KOH s...
Questions

Mathematics, 23.06.2021 08:00



Biology, 23.06.2021 08:00

Mathematics, 23.06.2021 08:00


Mathematics, 23.06.2021 08:00

Mathematics, 23.06.2021 08:00



Mathematics, 23.06.2021 08:00



Mathematics, 23.06.2021 08:10

Mathematics, 23.06.2021 08:10

History, 23.06.2021 08:10

English, 23.06.2021 08:10

Mathematics, 23.06.2021 08:10


Mathematics, 23.06.2021 08:10

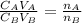
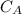 is the concentration of acid
is the concentration of acid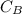 is the concentration of base
is the concentration of base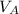 is the volume of acid
is the volume of acid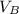 is the volume of base
is the volume of base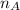 is the mole ratio of acid
is the mole ratio of acid 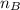 is the mole ratio of base
is the mole ratio of base