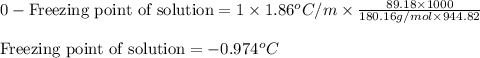Chemistry, 12.02.2020 18:31 gracebuffum
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freezing point of the solution. The density of the solution is 1.034 g/mL.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2


Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
At 298 K, the osmotic pressure of a glucose solution (C6H12O6 (aq)) is 12.1 atm. Calculate the freez...
Questions





History, 30.09.2019 03:00

Chemistry, 30.09.2019 03:00

Mathematics, 30.09.2019 03:00

Mathematics, 30.09.2019 03:00

English, 30.09.2019 03:00




History, 30.09.2019 03:00

History, 30.09.2019 03:00

Social Studies, 30.09.2019 03:00


Chemistry, 30.09.2019 03:00


Biology, 30.09.2019 03:00

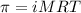
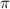 = osmotic pressure of the solution = 12.1 atm
= osmotic pressure of the solution = 12.1 atm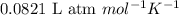
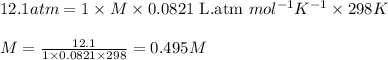
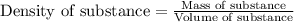
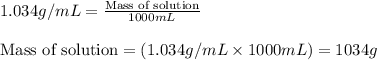
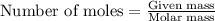
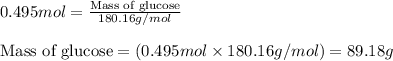
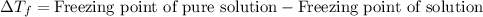
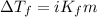
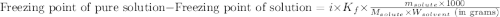
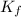 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m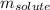 = Given mass of solute (glucose) = 89.18 g
= Given mass of solute (glucose) = 89.18 g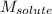 = Molar mass of solute (glucose) = 180.16 g/mol
= Molar mass of solute (glucose) = 180.16 g/mol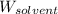 = Mass of solvent (water) = [1034 - 89.18] g = 944.82 g
= Mass of solvent (water) = [1034 - 89.18] g = 944.82 g