Chemistry, 12.02.2020 01:38 jennemylesp19oy5
If the percent yield for the following reaction is 75.0%, and 45.0 g of NO2 are consumed in the reaction, how many grams of nitric acid, HNO3(aq), are produced?
3 NO2(g) + H2O(l) ? 2 HNO3(aq) + NO(g)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
You know the right answer?
If the percent yield for the following reaction is 75.0%, and 45.0 g of NO2 are consumed in the reac...
Questions

Chemistry, 20.10.2020 03:01

Social Studies, 20.10.2020 03:01



Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


History, 20.10.2020 03:01


Computers and Technology, 20.10.2020 03:01



Mathematics, 20.10.2020 03:01

English, 20.10.2020 03:01




Arts, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

Chemistry, 20.10.2020 03:01

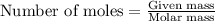 .....(1)
.....(1)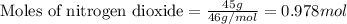
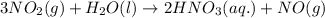
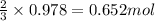 of nitric acid
of nitric acid