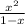Chemistry, 11.02.2020 23:59 brianna5626
In a chemical reaction, substrate molecule A is broken down to form one molecule of product B and one molecule of product C. The equilibrium constant, K, for this reaction is 0.5. If we start with a mixture containing only substrate A at a concentration of 1 M, what will be the concentration of A when the reaction reaches equilibrium?a. 0.125 Mb. 0.25 Mc. 0.333 Md. 0.5 Me. 0.667 M

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 19:00
How long does it take light from the nearest star other than the sun to reach earth? a) less than 1 second b) about 1 hour c) about 1 month d) about 4 years
Answers: 1
You know the right answer?
In a chemical reaction, substrate molecule A is broken down to form one molecule of product B and on...
Questions



Spanish, 30.06.2021 02:40

Mathematics, 30.06.2021 02:40

Health, 30.06.2021 02:40





History, 30.06.2021 02:40



Mathematics, 30.06.2021 02:40





Mathematics, 30.06.2021 02:40

Mathematics, 30.06.2021 02:40

Mathematics, 30.06.2021 02:40

![\frac{[B][C]}{A}](/tpl/images/0507/4816/fe80f.png)
![0.5 = \frac{[x][x]}{1 - x}](/tpl/images/0507/4816/07434.png)