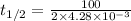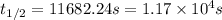Chemistry, 11.02.2020 21:27 litzyguzman13
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Determine the half-life t1/2} in units of seconds. Do not enter units with your numerical answer.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Dete...
Questions







Health, 03.10.2019 00:20



Mathematics, 03.10.2019 00:20


Biology, 03.10.2019 00:20


Mathematics, 03.10.2019 00:20

Mathematics, 03.10.2019 00:20



Mathematics, 03.10.2019 00:20

Mathematics, 03.10.2019 00:20


![\ln [A]=-kt+\ln [A_o]](/tpl/images/0507/1434/bdc3f.png)
![[A_o]](/tpl/images/0507/1434/dc622.png) = let initial concentration = 100
= let initial concentration = 100![[A]](/tpl/images/0507/1434/6aa06.png) = final concentration = 25
= final concentration = 25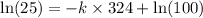
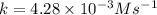
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0507/1434/b5b11.png)