
Chemistry, 11.02.2020 20:55 brummy309506
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H2(g) → 2NH3(g) Calculate the number of moles of hydrogen required to react with 0.0763 mole of nitrogen, and the number of moles of ammonia that will form.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1
You know the right answer?
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H...
Questions


English, 06.05.2020 08:10



Mathematics, 06.05.2020 08:10


Mathematics, 06.05.2020 08:10

Social Studies, 06.05.2020 08:10

History, 06.05.2020 08:10





Mathematics, 06.05.2020 08:10



Mathematics, 06.05.2020 08:10

Advanced Placement (AP), 06.05.2020 08:10

Biology, 06.05.2020 08:10

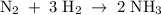
 3 moles of Hydrogen
3 moles of Hydrogen

