
Chemistry, 11.02.2020 04:24 jholland03
How much energy (heat) is required to convert 248 g of water from 0oC to 154oC? Assume that the water begins as a liquid, that the specific heat of water is 4.184 J/g. oC over the entire liquid range, that the specific heat of steam is 1.99 J/g. oC, and the heat of vaporization of water is 40.79 kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1


Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
How much energy (heat) is required to convert 248 g of water from 0oC to 154oC? Assume that the wate...
Questions



Mathematics, 25.12.2019 19:31



Mathematics, 25.12.2019 19:31

Mathematics, 25.12.2019 19:31

English, 25.12.2019 19:31

Chemistry, 25.12.2019 19:31





Mathematics, 25.12.2019 19:31





Chemistry, 25.12.2019 19:31

Mathematics, 25.12.2019 19:31

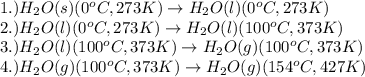
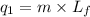
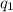 = amount of heat absorbed = ?
= amount of heat absorbed = ?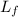 = latent heat of fusion = 334 J/g
= latent heat of fusion = 334 J/g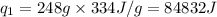
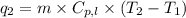
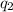 = amount of heat absorbed = ?
= amount of heat absorbed = ?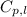 = specific heat of water = 4.184 J/g°C
= specific heat of water = 4.184 J/g°C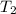 = final temperature =
= final temperature = 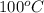
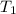 = initial temperature =
= initial temperature = 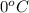
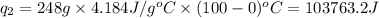
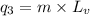
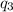 = amount of heat absorbed = ?
= amount of heat absorbed = ?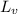 = latent heat of vaporization =
= latent heat of vaporization = 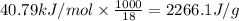 (Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)
(Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)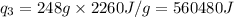
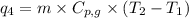
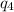 = amount of heat absorbed = ?
= amount of heat absorbed = ?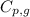 = specific heat of steam = 1.99 J/g°C
= specific heat of steam = 1.99 J/g°C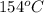
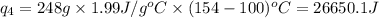
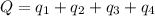
![Q=[84832+103763.2+560480+26650.1]J=775,725.3J=775.7kJ](/tpl/images/0506/1413/615e7.png)


