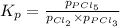Chemistry, 11.02.2020 00:56 cia196785920
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl2(g) equilibrium reaction arrow pcl5(g) a gas vessel is charged with a mixture of pcl3(g) and cl2(g), which is allowed to equilibriate at 450 k. at equilibrium the partial pressures of the three gases are ppcl3 = 0.124 atm, pcl2 = 0.157 atm, and ppcl5 = 1.30 atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
Phosphorus trichloride gas and chlorine gas react to form phosphorus pentachloride gas. pcl3(g) + cl...
Questions

Mathematics, 08.01.2020 09:31

English, 08.01.2020 09:31

History, 08.01.2020 09:31

History, 08.01.2020 09:31

Mathematics, 08.01.2020 09:31

Mathematics, 08.01.2020 09:31




Mathematics, 08.01.2020 09:31

Mathematics, 08.01.2020 09:31



Health, 08.01.2020 09:31

History, 08.01.2020 09:31


Mathematics, 08.01.2020 09:31

History, 08.01.2020 09:31


Biology, 08.01.2020 09:31

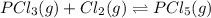
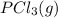 and
and 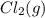 , which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are
, which is allowed to equilibriate at 450 K. At equilibrium the partial pressures of the three gases are 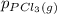 = 0.126 atm ,
= 0.126 atm , 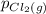 = 0.156 atm , and
= 0.156 atm , and 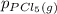 = 1.60 atm. What is the value of
= 1.60 atm. What is the value of 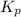 at this temperature?
at this temperature?