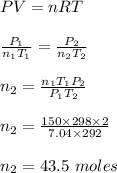Chemistry, 10.02.2020 05:39 Sonicawesomeness
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. After some argon has been used, the pressure is 2.00 MPa at a temperature of 19°C. What mass (g) of argon remains in the cylinder?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. A...
Questions



Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00



Biology, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Health, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

World Languages, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Social Studies, 12.12.2020 17:00

English, 12.12.2020 17:00