
Chemistry, 05.02.2020 21:43 Lindsay882
Suppose a 1.30 g nugget of pure gold has zero net charge. what would be its net charge after it has 1.68% of its electrons removed?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1

Chemistry, 23.06.2019 13:30
What happens to acetone molecules when you add heat to a beaker of liquid acetone?
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1
You know the right answer?
Suppose a 1.30 g nugget of pure gold has zero net charge. what would be its net charge after it has...
Questions

Computers and Technology, 19.02.2020 17:36


Computers and Technology, 19.02.2020 17:36







Mathematics, 19.02.2020 17:37




Computers and Technology, 19.02.2020 17:37

Mathematics, 19.02.2020 17:37



Computers and Technology, 19.02.2020 17:37

Computers and Technology, 19.02.2020 17:37

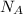 = 0.0066 × 6.02 × 10²³ = 3.97 × 10²¹ atoms
= 0.0066 × 6.02 × 10²³ = 3.97 × 10²¹ atoms 


