Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
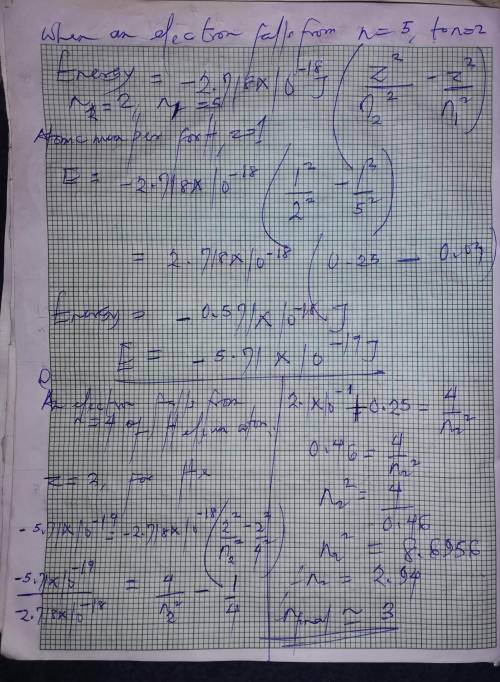
When the excited electron in a hydrogen atom falls from to , a photon of blue light is emitted. if a...
Questions

Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00



Chemistry, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00





Mathematics, 01.05.2021 01:00




Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00