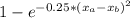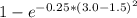Chemistry, 28.01.2020 20:48 Tabbicat021
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding. the electronegativities for and are 1.5 and 3.0 respectively. calculate the fraction of the bonding that is ionic. (enter your answer to three significant figures.) =

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

You know the right answer?
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding...
Questions


Biology, 01.02.2020 00:57

History, 01.02.2020 00:57

Mathematics, 01.02.2020 00:57






Biology, 01.02.2020 00:57




Social Studies, 01.02.2020 00:57

Spanish, 01.02.2020 00:57

English, 01.02.2020 00:57



History, 01.02.2020 00:57

English, 01.02.2020 00:57