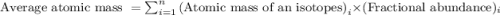Chemistry, 24.01.2020 01:31 ashkids7178
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a mass of 64.93 amu. from the atomic weight of cu = 63.54 one can conclude that:
- both isotopes have the same percent natural abundance
- copper-65 has the highest percent natural abundance
- most copper atoms have an atomic mass of 63.54
- copper-63 has the highest percent natural abundance

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
The element copper has two stable isotopes, copper-63 with a mass of 62.93 amu and copper-65 with a...
Questions



Social Studies, 27.07.2019 12:40


Biology, 27.07.2019 12:40

Biology, 27.07.2019 12:40



Physics, 27.07.2019 12:50


History, 27.07.2019 12:50



Physics, 27.07.2019 12:50



Health, 27.07.2019 12:50


Arts, 27.07.2019 12:50