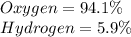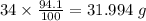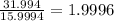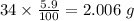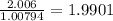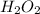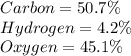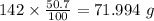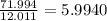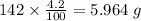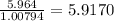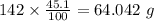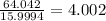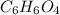Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar mass = 34g
b) 50.7% carbon, 4.2% hydrogen, and 45.1% oxygen; molar mass = 142g
(would greatly appreciated if someone could explain the process, also use the correct amount of significant digits)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar...
a) 94.1% oxygen and 5.9% hydrogen; molar...
Questions


Mathematics, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40



Mathematics, 04.12.2020 03:40

English, 04.12.2020 03:40


Mathematics, 04.12.2020 03:40



English, 04.12.2020 03:40






Mathematics, 04.12.2020 03:40

Mathematics, 04.12.2020 03:40

 molecules.
molecules.