
Chemistry, 20.01.2020 21:31 maystrenko53
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. an industrial chemist studying this reaction fills a 500 ml flask with 4.8 atm of ammonia gas and 1.9 atm of oxygen gas at 33 degress celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be 0.63 atm. calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2
You know the right answer?
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its r...
Questions


Computers and Technology, 28.10.2019 22:31



History, 28.10.2019 22:31



English, 28.10.2019 22:31






Social Studies, 28.10.2019 22:31





Mathematics, 28.10.2019 22:31

Biology, 28.10.2019 22:31

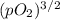 ]
]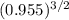 ]
]

