
Chemistry, 20.01.2020 03:31 tatianaflores9040
The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → cacl_2 (aq) + h_2 (g)
if 500.0 g of calcium are added to an excess of hci, what volume of h_2 is produced?
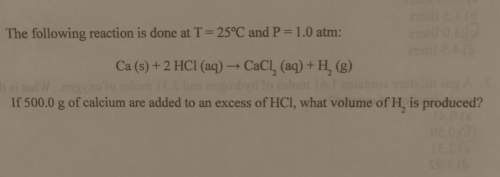

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the molality of a solution that has 4 mol of kci in 0.800 kg of water
Answers: 3

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
The following reaction is done at
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → c...
t = 25°c and p= 1.0 atm:
ca (s) + 2 hcl (aq) → c...
Questions


History, 26.06.2019 10:00

Mathematics, 26.06.2019 10:00







History, 26.06.2019 10:00

SAT, 26.06.2019 10:00


Mathematics, 26.06.2019 10:00

Arts, 26.06.2019 10:00


Mathematics, 26.06.2019 10:00

History, 26.06.2019 10:00

History, 26.06.2019 10:00





