
Chemistry, 05.10.2019 10:30 sitstareplay7073
Given the following balanced reaction between nitrogen gas and oxygen gas to produce nitrous oxide gas, how many moles of nitrous oxide gas are produced from 1.65 moles of nitrogen gas?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
Given the following balanced reaction between nitrogen gas and oxygen gas to produce nitrous oxide g...
Questions

History, 21.02.2020 19:57


Mathematics, 21.02.2020 19:57

Mathematics, 21.02.2020 19:57

Chemistry, 21.02.2020 19:57



Biology, 21.02.2020 19:57

Physics, 21.02.2020 19:57

History, 21.02.2020 19:57

Mathematics, 21.02.2020 19:57


Physics, 21.02.2020 19:58






Mathematics, 21.02.2020 19:58

English, 21.02.2020 19:58

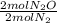 = 1,65 mol N₂O
= 1,65 mol N₂O

