The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the p...

Chemistry, 09.01.2020 06:31 jessixa897192
The distribution constant for a molecule x between n-hexane and water
is 13.5. calculate the percent of x remaining in the water phase that
was originally 0.0100 m in z after extraction of 50.0 ml of water with
4 extractions of 10.0 ml portions of n-hexane.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Questions



Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00


Geography, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00


Mathematics, 11.02.2021 01:00

Social Studies, 11.02.2021 01:00

History, 11.02.2021 01:00

Mathematics, 11.02.2021 01:00





English, 11.02.2021 01:00

Physics, 11.02.2021 01:00

![[X]_{i} = (\frac{V_{aq}}{V_{or}KD + V_{aq}})^{i} \cdot [X_{0}]](/tpl/images/0448/1496/650eb.png)
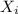 : is the concentration of A remaining in aqueous solution
: is the concentration of A remaining in aqueous solution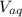 : is the volume of water
: is the volume of water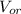 : is the volume of the organic solvent
: is the volume of the organic solvent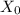 : is the original concentration of X
: is the original concentration of X![[X]_{i} = (\frac{50 mL}{10 mL \cdot 13.5 + 50 mL})^{4} \cdot 0.01 M = 5.34 \cdot 10^{-5} M](/tpl/images/0448/1496/4cebe.png)
![[X]_{i} = 5.34 \cdot 10^{-5} \cdot 100 \% = 0.005 \%](/tpl/images/0448/1496/48d0a.png)


