
Chemistry, 23.12.2019 20:31 serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1


Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions








English, 14.12.2019 00:31



English, 14.12.2019 00:31

Mathematics, 14.12.2019 00:31

Physics, 14.12.2019 00:31


Social Studies, 14.12.2019 00:31





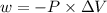
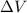 is the change in volume
is the change in volume
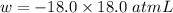
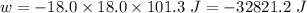 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)

