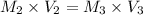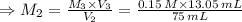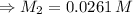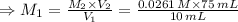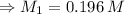Chemistry, 20.12.2019 02:31 halimomohamed
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer flask. an excess of ki solution was added. indicator was added and the solution was diluted with h2o to a total volume of 75 ml. for rxn 2, the solution from rxn 1 was titrated with 0.15 m na2s2o3. the equivalence point of the titration was reached when 13.05 ml of na2s2o3 had been added. what is the molar concentration of cu2+ in the original 10.0 ml solution?

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1

Chemistry, 23.06.2019 16:00
Challenge question: this question is worth 6 points. as you saw in problem 9 we can have species bound to a central metal ion. these species are called ligands. in the past we have assumed all the d orbitals in some species are degenerate; however, they often are not. sometimes the ligands bound to a central metal cation can split the d orbitals. that is, some of the d orbitals will be at a lower energy state than others. ligands that have the ability to cause this splitting are called strong field ligands, cnâ’ is an example of these. if this splitting in the d orbitals is great enough electrons will fill low lying orbitals, pairing with other electrons in a given orbital, before filling higher energy orbitals. in question 7 we had fe2+, furthermore we found that there were a certain number (non-zero) of unpaired electrons. consider now fe(cn)6 4â’: here we also have fe2+, but in this case all the electrons are paired, yielding a diamagnetic species. how can you explain this?
Answers: 2

Chemistry, 23.06.2019 17:30
With carbon dioxide what phase change take place when the temperature decreases from -40c to -80c at 2 atm
Answers: 2
You know the right answer?
For rxn 1, 10.0 ml of a cu2+ solution of unknown concentration was placed in a 250 ml erlenmeyer fla...
Questions


English, 08.10.2019 20:30