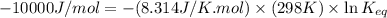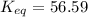Chemistry, 20.12.2019 01:31 maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 3

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions


Mathematics, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01


Mathematics, 21.09.2020 07:01

History, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01

Spanish, 21.09.2020 07:01

Biology, 21.09.2020 07:01

Mathematics, 21.09.2020 07:01



Mathematics, 21.09.2020 07:01


Chemistry, 21.09.2020 07:01



Mathematics, 21.09.2020 07:01

Physics, 21.09.2020 07:01

History, 21.09.2020 07:01

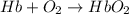 ;
; 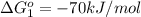
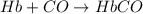 ;
; 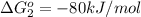
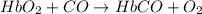 ;
; 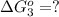
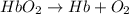 ;
; 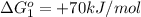
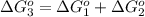
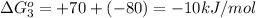
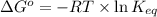
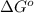 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol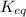 = equilibrium constant = ?
= equilibrium constant = ?