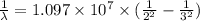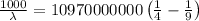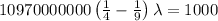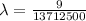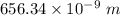Chemistry, 18.12.2019 01:31 tylermdons
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation, ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
a) calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3 . round your answer to significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
The energy of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -r...
e= -r...
Questions


Mathematics, 29.11.2019 12:31


Mathematics, 29.11.2019 12:31

Mathematics, 29.11.2019 12:31


History, 29.11.2019 12:31

Mathematics, 29.11.2019 12:31


Mathematics, 29.11.2019 12:31


Mathematics, 29.11.2019 12:31

Mathematics, 29.11.2019 12:31

History, 29.11.2019 12:31



Mathematics, 29.11.2019 12:31

Mathematics, 29.11.2019 12:31

Mathematics, 29.11.2019 12:31

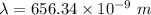
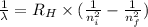
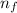 = 3
= 3
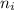 = 2
= 2