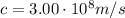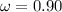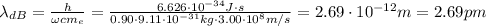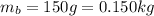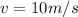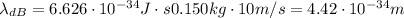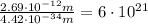Chemistry, 17.12.2019 07:31 ddwithdadarco
Calculate "de broglie" wavelength for each of the following, and use your numerical answers the to explain why macroscopic (large) objects are not ordinarily discussed in terms of their "wave-like" properties. a. an electron moving at .90 times the speed of light.
b. a 150-g ball moving at a speed of 10.m/s

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Calculate "de broglie" wavelength for each of the following, and use your numerical answers the to e...
Questions

Mathematics, 29.08.2019 00:30

Chemistry, 29.08.2019 00:30



Chemistry, 29.08.2019 00:30


History, 29.08.2019 00:30

Mathematics, 29.08.2019 00:30

Mathematics, 29.08.2019 00:30



Mathematics, 29.08.2019 00:30



History, 29.08.2019 00:30

Social Studies, 29.08.2019 00:30

Social Studies, 29.08.2019 00:30

Biology, 29.08.2019 00:30

Chemistry, 29.08.2019 00:30

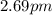
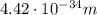
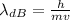
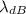 is the de Broglie wavelength (in m);
is the de Broglie wavelength (in m);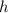 is the Planck's constant,
is the Planck's constant, 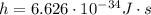 ;
;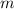 is mass (in kg);
is mass (in kg);![v[tex] is velocity (in m/s).a. We need to know the mass of an electron here:[tex]m_e=9.11\cdot10^{-31} kg](/tpl/images/0422/1849/0b8f1.png)