
Chemistry, 11.12.2019 21:31 keniaguevara32
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reactions and given δh values. ch4(g)+cl2(g)→ch3cl(g)+hcl(g), δh=−99.60 kj ch3cl(g)+cl2(g)→ch2cl2(g)+hcl(g), δh=−105.8 kj express your answer to four significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Calculate δhrxn for the following reaction: ch4(g)+2cl2(g)→ch2cl2(g)+2hcl(g) use the following reac...
Questions




Mathematics, 09.04.2021 18:00

Mathematics, 09.04.2021 18:00

Mathematics, 09.04.2021 18:00






Biology, 09.04.2021 18:00

Mathematics, 09.04.2021 18:00

Social Studies, 09.04.2021 18:00


Mathematics, 09.04.2021 18:00


Physics, 09.04.2021 18:00


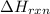 for the reaction is -205.4 kJ.
for the reaction is -205.4 kJ.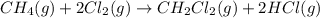
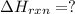
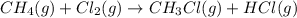
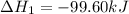
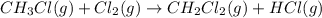
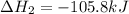
![\Delta H_{rxn}=[1\times \Delta H_1]+[1\times \Delta H_2]](/tpl/images/0414/0614/6e774.png)
![\Delta H_{rxn}=[(1\times (-99.60))+(1\times (-105.8))]=-205.4kJ](/tpl/images/0414/0614/3a575.png)


