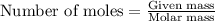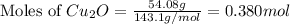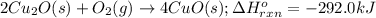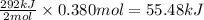Chemistry, 11.12.2019 05:31 Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1


Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions

Mathematics, 12.04.2020 06:24






Mathematics, 12.04.2020 06:24

Mathematics, 12.04.2020 06:24




Mathematics, 12.04.2020 06:24

Computers and Technology, 12.04.2020 06:24






Mathematics, 12.04.2020 06:25

Chemistry, 12.04.2020 06:25

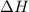 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ