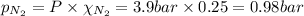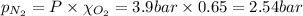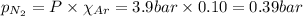Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
Amixture of n2, o2, and ar has mole fractions of 0.25, 0.65, and 0.10, respectively. what is the pre...
Questions


Social Studies, 01.09.2019 23:20

Mathematics, 01.09.2019 23:20

Chemistry, 01.09.2019 23:20


Mathematics, 01.09.2019 23:20

English, 01.09.2019 23:20


Mathematics, 01.09.2019 23:20

History, 01.09.2019 23:20




Mathematics, 01.09.2019 23:20


Biology, 01.09.2019 23:20


Biology, 01.09.2019 23:20

Social Studies, 01.09.2019 23:20

Business, 01.09.2019 23:20

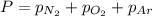
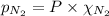
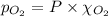
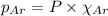
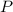 = total pressure = 3.9 bar
= total pressure = 3.9 bar 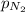 = partial pressure of nitrogen gas
= partial pressure of nitrogen gas 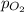 = partial pressure of oxygen gas
= partial pressure of oxygen gas 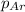 = partial pressure of argon gases
= partial pressure of argon gases 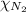 = Mole fraction of nitrogen gas = 0.25
= Mole fraction of nitrogen gas = 0.25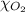 = Mole fraction of oxygen gas = 0.65
= Mole fraction of oxygen gas = 0.65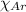 = Mole fraction of argon gases = 0.10
= Mole fraction of argon gases = 0.10