
Chemistry, 10.12.2019 04:31 sbudlove2838
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h...
Questions

English, 03.08.2019 04:00





Mathematics, 03.08.2019 04:00



English, 03.08.2019 04:00


Chemistry, 03.08.2019 04:00



Social Studies, 03.08.2019 04:00


Geography, 03.08.2019 04:00

Computers and Technology, 03.08.2019 04:00

Physics, 03.08.2019 04:00


Computers and Technology, 03.08.2019 04:00

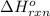 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.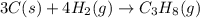
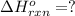
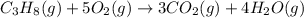
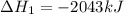
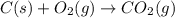
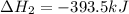 ( × 3)
( × 3) 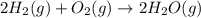
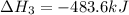 ( × 2)
( × 2) ![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0411/2485/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times (-(-2043))+(3\times (-393.5))+(2\times (-483.6))]=-104.7kJ](/tpl/images/0411/2485/eafbb.png)


