
Chemistry, 07.12.2019 02:31 jdvazquez18p7a7vs
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bridge.• a fe electrode in 1.0 m fecl2 solution• a sn electrode in 1.0 m sn(no3)2 solutionwhen the cell is running spontaneously, which choice includes only true statements and no false ones? question 1 options: a. the tin electrode loses mass and the tin electrode is the cathode. b. the tin electrode gains mass and the tin electrode is the cathode. c. the iron electrode gains mass and the iron electrode is the anode. d. the iron electrode loses mass and the iron electrode is the cathode.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bri...
Questions

Chemistry, 10.05.2021 19:00





English, 10.05.2021 19:00

History, 10.05.2021 19:00




Mathematics, 10.05.2021 19:00





Mathematics, 10.05.2021 19:00




Mathematics, 10.05.2021 19:00

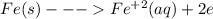
 is higher than tin(ii) ion,
is higher than tin(ii) ion, 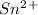 in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a
in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a 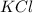 salt bridge:So, an Fe electrode in 1.0 M
salt bridge:So, an Fe electrode in 1.0 M 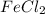 solution and a
solution and a 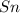 electrode in 1.0 M
electrode in 1.0 M 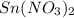 solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The
solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The 

