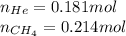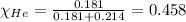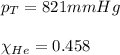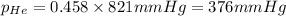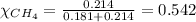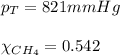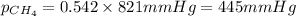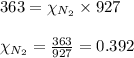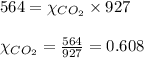Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of helium and 3.43 grams of methane. what is the partial pressure of each gas in the mixture?
phe = mm hg
pch4 = mm hg
2.) a mixture of nitrogen and carbon dioxide gases contains nitrogen at a partial pressure of 363 mm hg and carbon dioxide at a partial pressure of 564 mm hg. what is the mole fraction of each gas in the mixture?
xn2 =
xco2 =

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Amixture of helium and methane gases, at a total pressure of 821 mm hg, contains 0.723 grams of heli...
Questions



Mathematics, 10.04.2020 20:20

English, 10.04.2020 20:20



Computers and Technology, 10.04.2020 20:20

Mathematics, 10.04.2020 20:20


Mathematics, 10.04.2020 20:20

Mathematics, 10.04.2020 20:20





Mathematics, 10.04.2020 20:20

Spanish, 10.04.2020 20:20

History, 10.04.2020 20:20


Mathematics, 10.04.2020 20:20

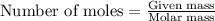 .....(1)
.....(1)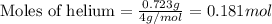
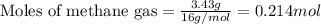
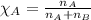 .......(2)
.......(2)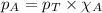 ......(3)
......(3)