
Chemistry, 06.12.2019 05:31 smkw04p3ao0n
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hcl(g) calculate the equilibrium concentration of hcl when 0.564 moles of nh4cl(s) are introduced into a 1.00 l vessel at 548 k.[hcl] = m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hc...
Questions

English, 04.10.2021 03:00

Mathematics, 04.10.2021 03:00

Mathematics, 04.10.2021 03:00

Social Studies, 04.10.2021 03:00

Mathematics, 04.10.2021 03:00

Biology, 04.10.2021 03:00

Mathematics, 04.10.2021 03:00


Mathematics, 04.10.2021 03:00


Biology, 04.10.2021 03:10



Biology, 04.10.2021 03:10


English, 04.10.2021 03:10

Computers and Technology, 04.10.2021 03:10

Chemistry, 04.10.2021 03:10


Mathematics, 04.10.2021 03:10

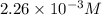
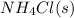 = 0.564 moles
= 0.564 moles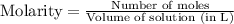
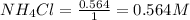
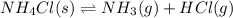
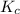 for above equation follows:
for above equation follows:![K_c=[NH_3][HCl]](/tpl/images/0405/9498/72be1.png)
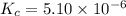
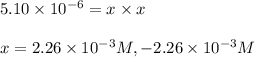
![[HCl]=2.26\times 10^{-3}M](/tpl/images/0405/9498/283fd.png)


