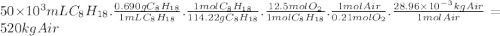Chemistry, 05.12.2019 19:31 mayamcmillan11
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (approximately 13.2 gallons), determine mass of air in kg required to combustion it. you can use isooctane c8h18 to approximate gasoline

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (a...
Questions

Mathematics, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01


History, 03.09.2020 20:01

English, 03.09.2020 20:01


Mathematics, 03.09.2020 20:01

Mathematics, 03.09.2020 20:01


History, 03.09.2020 20:01

Health, 03.09.2020 20:01




Biology, 03.09.2020 20:01

Arts, 03.09.2020 20:01