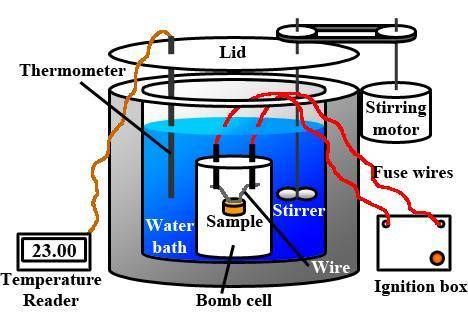
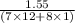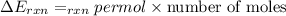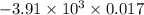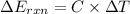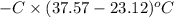When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87∘c to 38.13∘c. find δerxn for the reaction in kj/mol hexane. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kj/∘c. express your answer in kilojoules per mole to three significant figures.2. the combustion of toluene has a δerxn of –3.91×103 kj/mol. when 1.55 g of toluene (c7h8) undergoes combustion in a bomb calorimeter, the temperature rises from 23.12∘c to 37.57∘c. find the heat capacity of the bomb calorimeter. express the heat capacity in kilojoules per degree celsius to three significant

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature ri...
Questions

Mathematics, 01.10.2019 06:30






Biology, 01.10.2019 06:30



English, 01.10.2019 06:30


History, 01.10.2019 06:30



Mathematics, 01.10.2019 06:30

Geography, 01.10.2019 06:30

Mathematics, 01.10.2019 06:30

Mathematics, 01.10.2019 06:30


Health, 01.10.2019 06:30

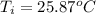 ,
, 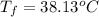
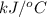
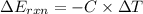
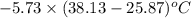
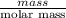
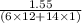
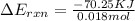
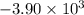 kJ/mol
kJ/mol
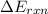 for the reaction in kJ/mol hexane is
for the reaction in kJ/mol hexane is