
Chemistry, 03.12.2019 21:31 courtnicollee
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the solution, which converts fe(ii) to insoluble fe(iii): 4fe(oh)+(aq)+ 4oh-(aq)+o2(g)+2h2o(l)=4fe(oh)3(s)h ow many grams of o2 are consumed to precipitate all of the iron in 85ml of 0.075 m fe(ii).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Fe(ii) can be precipitated from a slightly basic (aq) solution by bubbling oxygen through the soluti...
Questions



Biology, 30.09.2019 11:00

Mathematics, 30.09.2019 11:00


English, 30.09.2019 11:00

Spanish, 30.09.2019 11:00

Mathematics, 30.09.2019 11:00

Geography, 30.09.2019 11:00

Physics, 30.09.2019 11:00








Social Studies, 30.09.2019 11:10

Mathematics, 30.09.2019 11:10

History, 30.09.2019 11:10

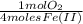 = 1,6x10⁻³ moles of O₂(g)
= 1,6x10⁻³ moles of O₂(g)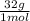 = 0,051g of O₂ are consumed
= 0,051g of O₂ are consumed

