
Chemistry, 03.12.2019 04:31 applereams
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxygen (o2).
4al + 3o2 > 2al2o3
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 must react to form 3.80 mol of al2o3?
60.8
81.1
122
182

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
You know the right answer?
The chemical equation below shows the formation of aluminum oxide (al2o3) from aluminum (al) and oxy...
Questions



Mathematics, 29.08.2020 19:01

Computers and Technology, 29.08.2020 19:01





Business, 29.08.2020 19:01




Mathematics, 29.08.2020 19:01

Advanced Placement (AP), 29.08.2020 19:01






Business, 29.08.2020 19:01

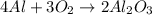
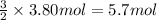 of oxygen gas.
of oxygen gas.

