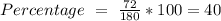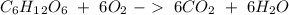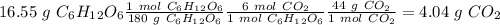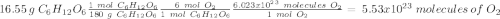Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems. we were provided 16.55 g of glucose. calculate:
a) the mass percent of carbon in glucose.
b) the mass of co2 produced by the combustion of 16.55 g glucose with sufficient oxygen gas.
c) how many oxygen molecules needed for the completely combustion of 16.55 g glucose?

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems....
Questions


Mathematics, 27.01.2021 04:40


Social Studies, 27.01.2021 04:40

Mathematics, 27.01.2021 04:40

Mathematics, 27.01.2021 04:40


Mathematics, 27.01.2021 04:40

Mathematics, 27.01.2021 04:40

Mathematics, 27.01.2021 04:40



Mathematics, 27.01.2021 04:40





Mathematics, 27.01.2021 04:50


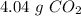
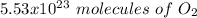
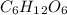 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.