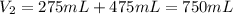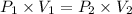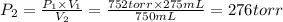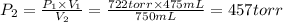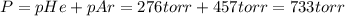A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml contains pure argon at a pressure of 722 torr. if the two flasks are connected through a stopcock and the stopcock is opened, what is the partial pressure of each gas and the total pressure.?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
A275-ml flask contains pure helium at a pressure of 752 torr. a second flask with a volume of 475 ml...
Questions

Mathematics, 18.03.2022 17:50




English, 18.03.2022 17:50

Mathematics, 18.03.2022 17:50






Mathematics, 18.03.2022 17:50





English, 18.03.2022 18:00


Business, 18.03.2022 18:00

Advanced Placement (AP), 18.03.2022 18:00