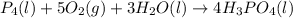Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: p4(l)+5o2(g-2 p20s(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acld p20(9)+3 h200 2h, po40) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions


Mathematics, 09.10.2019 02:00

Chemistry, 09.10.2019 02:00


History, 09.10.2019 02:00







Mathematics, 09.10.2019 02:00



Chemistry, 09.10.2019 02:00





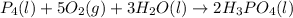
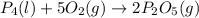 ......(1)
......(1)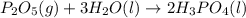 .......(2)
.......(2)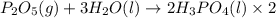
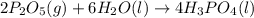 .......(3)
.......(3)