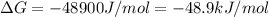Chemistry, 21.11.2019 04:31 alexreddin3127
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp (aq)+ h20 (l) -> adp (aq) + hpo4 (negative two overall charge) (aq).
for which ? g�rxn = �30.5 kj/mol at 37.0 �c and ph 7.0. calculate the value of ? grxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.80 mm, and [hpo42�] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Mathematics, 11.02.2021 22:00



Mathematics, 11.02.2021 22:00

Social Studies, 11.02.2021 22:00




Chemistry, 11.02.2021 22:00

Mathematics, 11.02.2021 22:00




Mathematics, 11.02.2021 22:00

Mathematics, 11.02.2021 22:00

Chemistry, 11.02.2021 22:00

Mathematics, 11.02.2021 22:00



Mathematics, 11.02.2021 22:00

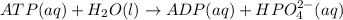
![[HPO_4^{2-}] = 5.0 mM=0.005 M](/tpl/images/0384/0544/f1ef4.png)
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}=\frac{0.0008 M\times 0.005 M}{0.005 M}=0.0008](/tpl/images/0384/0544/0fdb9.png)
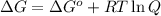
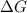 = Gibbs free energy at given conditions
= Gibbs free energy at given conditions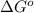 = Gibbs free energy at equilibrium=-30.5 kJ/mol
= Gibbs free energy at equilibrium=-30.5 kJ/mol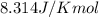
![37^oC=[273.15+37]K=310.15 K](/tpl/images/0384/0544/5ce7f.png)
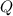 = reaction quotient at 37°C = 0.0008
= reaction quotient at 37°C = 0.0008![\Delta G=-30500 J/mol+(8.314J/Kmol)\times 310.15 K\times \ln [0.0008]](/tpl/images/0384/0544/d6332.png)