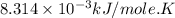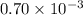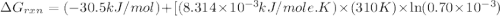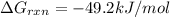Chemistry, 21.11.2019 04:31 juju323261
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp(aq)+h2o(l)⟶adp(aq)+hpo2−4(aq) for which δ∘rxn=−30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ in a biological cell in which [atp]=5.0 mm, [adp]=0.70 mm, and [hpo2−4]=5.0 mm.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions



Mathematics, 21.05.2020 07:59

English, 21.05.2020 07:59


Social Studies, 21.05.2020 07:59

Advanced Placement (AP), 21.05.2020 07:59

Mathematics, 21.05.2020 07:59






Biology, 21.05.2020 07:59






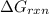 is -49.2 kJ/mol
is -49.2 kJ/mol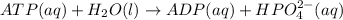
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0384/0553/ccdf0.png)
![[ATP]](/tpl/images/0384/0553/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0384/0553/68360.png) = 0.70 mM
= 0.70 mM![[HPO_4^{2-}]](/tpl/images/0384/0553/c0ca9.png) = 5.0 mM
= 5.0 mM
 ............(1)
............(1)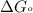 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol